Combretastatin A-1
CAS No. 109971-63-3
Combretastatin A-1( —— )
Catalog No. M34890 CAS No. 109971-63-3
Combretastatin A-1 is a potent microtubule inhibitor with anti-tumour and anti-vascular activity, acting through microtubule protein depolymerisation-mediated inactivation of AKT to inhibit the Wnt/β-catenin pathway, and can be used to study hepatocellular carcinoma.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 53 | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameCombretastatin A-1
-
NoteResearch use only, not for human use.
-
Brief DescriptionCombretastatin A-1 is a potent microtubule inhibitor with anti-tumour and anti-vascular activity, acting through microtubule protein depolymerisation-mediated inactivation of AKT to inhibit the Wnt/β-catenin pathway, and can be used to study hepatocellular carcinoma.
-
DescriptionCombretastatin A-1 is a microtubule polymerization inhibitor that binds to the colchicine-binding site of tubulin. Combretastatin A-1 inhibits the Wnt/β-catenin pathway through tubulin depolymerization mediated AKT deactivation. Combretastatin A-1 exhibits anti-tumor and anti-vascular effects.
-
In VitroCombretastatin A-1 (72 h) inhibits the growth of various tumor cell lines in vitro, including HepG2, SMMC-7721, Hepa 1-6, LM-3, Bel-7402, Huh7, BGC-803, MDA-MB-231, MCF-7, A375, NCI-1975, CT-26, HT-29, A549 cells (IC50=9.2, 12.8, 32.9, 33.8, 38.4, 728.2, 12.2, 17.6, 46.0, 61.0, 256.3, 1075.0, 2082.0, 2247.0 nM, respectively).Combretastatin A-1 (1-10 nM; 24 h) induces apoptosis by microtubule depolymerization-induced AKT inactivation and the removal of GSK-3β inhibition in HepG2 cells. Combretastatin A-1 (1-50?nM; 6 h) decreases the mitochondrial membrane potential (MMP) of HepG2 cells. Combretastatin A-1 shows dose-dependently ROS accumulation in HepG2 cells..Western Blot Analysis Cell Line:HepG2 cells Concentration:1, 5, 10 nM Incubation Time:24 hours Result:Significantly decreased Mcl-1 expression, but the Bcl-2 level was unchanged.Reduced p-GSK 3β (Ser9) without altering total GSK-3β protein levels, indicating an activation of GSK-3β.Reduced AKT phosphorylation on Ser473 without an obvious change in the total AKT protein levels.
-
In VivoCombretastatin A-1 (1-4?mg/kg; i.v. every other day for 4 weeks) significantly reduces the tumor volume in HepG2 subcutaneous xenograft model.Combretastatin A-1 (2 mg/kg; every other day for 21 days) shows enhanced apoptosis in orthotopic hepatocellular carcinoma mouse model.Animal Model:Male athymic BALB/c nu/nu mice (16-18 g; 4-6 weeks old) were inoculated with HepG2 cells Dosage:1, 2, 4?mg/kg Administration:I.v. every other day for 4 weeks Result:Resulted in a significant tumor volume reduction at the dose of 2?mg/kg or 4?mg/kg.
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorMicrotubule Associated | Akt
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number109971-63-3
-
Formula Weight332.35
-
Molecular FormulaC18H20O6
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : ≥ 100 mg/mL (300.89 mM )
-
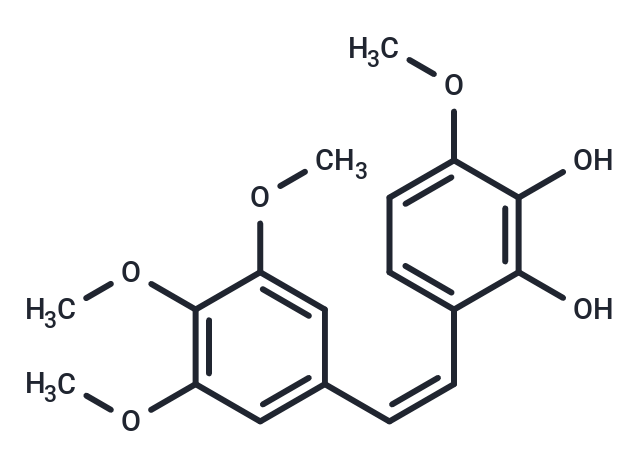
SMILESCOc1ccc(\C=C/c2cc(OC)c(OC)c(OC)c2)c(O)c1O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Pettit GR, et, al. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J Nat Prod. Jan-Feb 1987;50(1):119-31.?
molnova catalog



related products
-
Cephalandole B
cephalandole B, an indole alkaloid isolated from cyanide, significantly inhibited IL-17A gene expression and suppressed IL-17A luciferase reporter in Jukat cells in a dose-dependent manner.
-
Bis(2-ethylhexyl)hex...
Bis(2-ethylhexyl)hexanedioate (NSC 56775) is a diester produced by the formal condensation of the carboxyl group of adipic acid with 2-ethylhexan-1-ol, and is used as a plasticizer in the preparation of various polymers.
-
Tos-Gly-Pro-Arg-ANBA...
Tos-Gly-Pro-Arg-ANBA-IPA is a chromogenic peptide substrate. Tos-Gly-Pro-Arg-ANBA-IPA is used for the rapid and specific photometric assay of recombinant hirudin (r-hirudin).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


